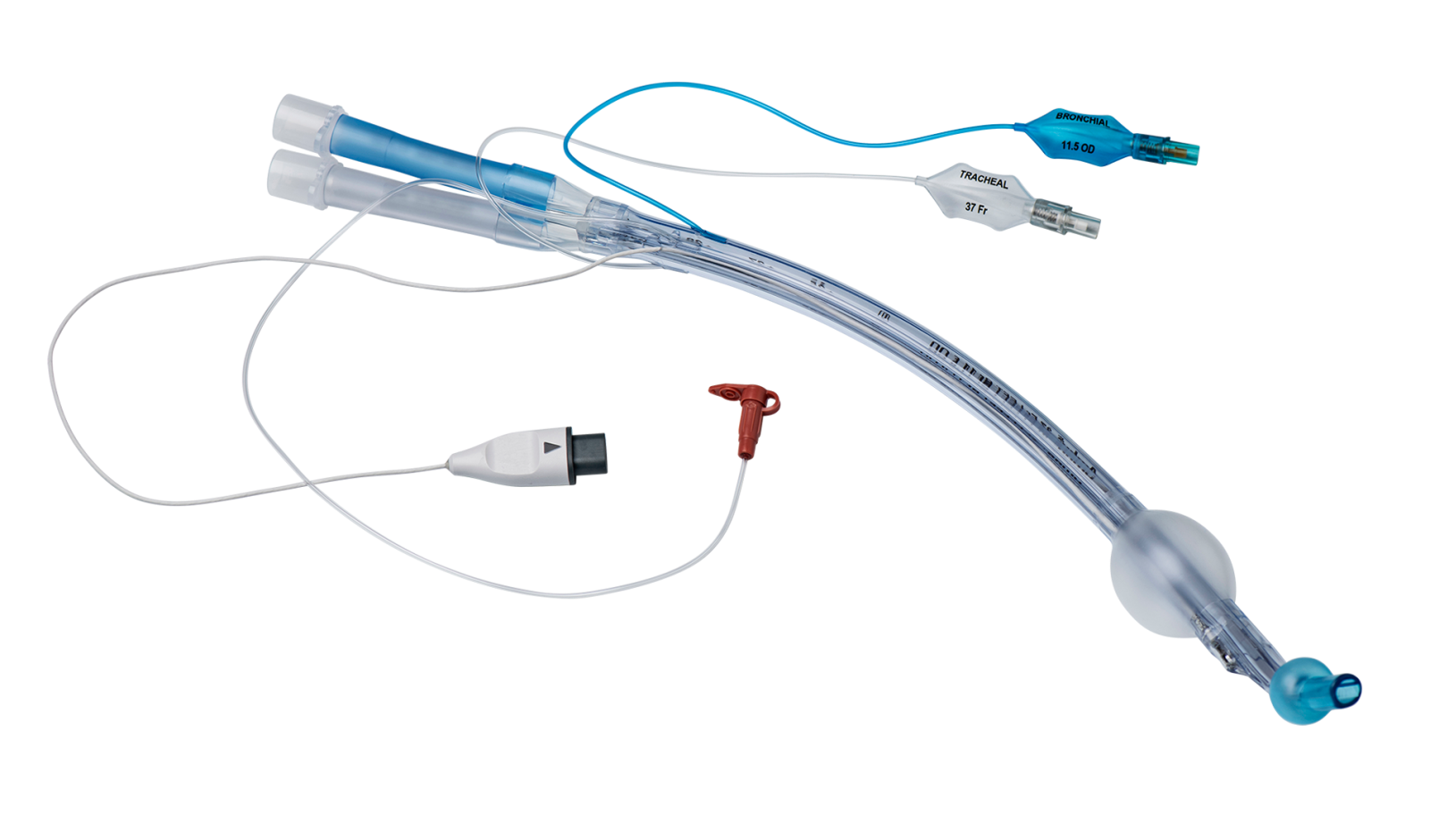
A safe and cost-effective way to perform One Lung Ventilation
A safe and cost-effective way to perform One Lung Ventilation
VivaSight 2 DLT is a sterile, single-use double lumen endobronchial tube used to isolate the left or right lung of a patient in One Lung Ventilation (OLV) procedures.
Increases patient safety
VivaSight 2 DLT is similar in design to conventional double lumen tubes, but it has a built-in video camera that connects to an Ambu monitor.
Continuous visualization enables you to follow tube placement and patient airway in real time throughout the entire OLV procedure. This allows you to immediately detect and correct malpositioning and dislocation of the DLT.
Improves procedure workflow
Continuous visualization of the tube and patient airway can positively affect patient management and procedure workflow because it:
- Enables faster intubation time than with conventional DLTs
- Facilitates correct tube placement
- Significantly reduces the need to use a bronchoscope
- Facilitates immediate detection and correction of tube dislodgement
A cost-effective alternative for OLV
A health-economic study (Larsen et al) indicates that using a DLT with an integrated video camera offers a cost-effective alternative for OLV procedures.
The study compared the costs and effects of a conventional DLT used in combination with a reusable bronchoscope to video-enabled DLT (VivaSight) alone.
KEY BENEFITS
Continuous visualization
FAST & EFFECTIVE
Comprehensive Ambu OLV solution
Perform procedures confidently
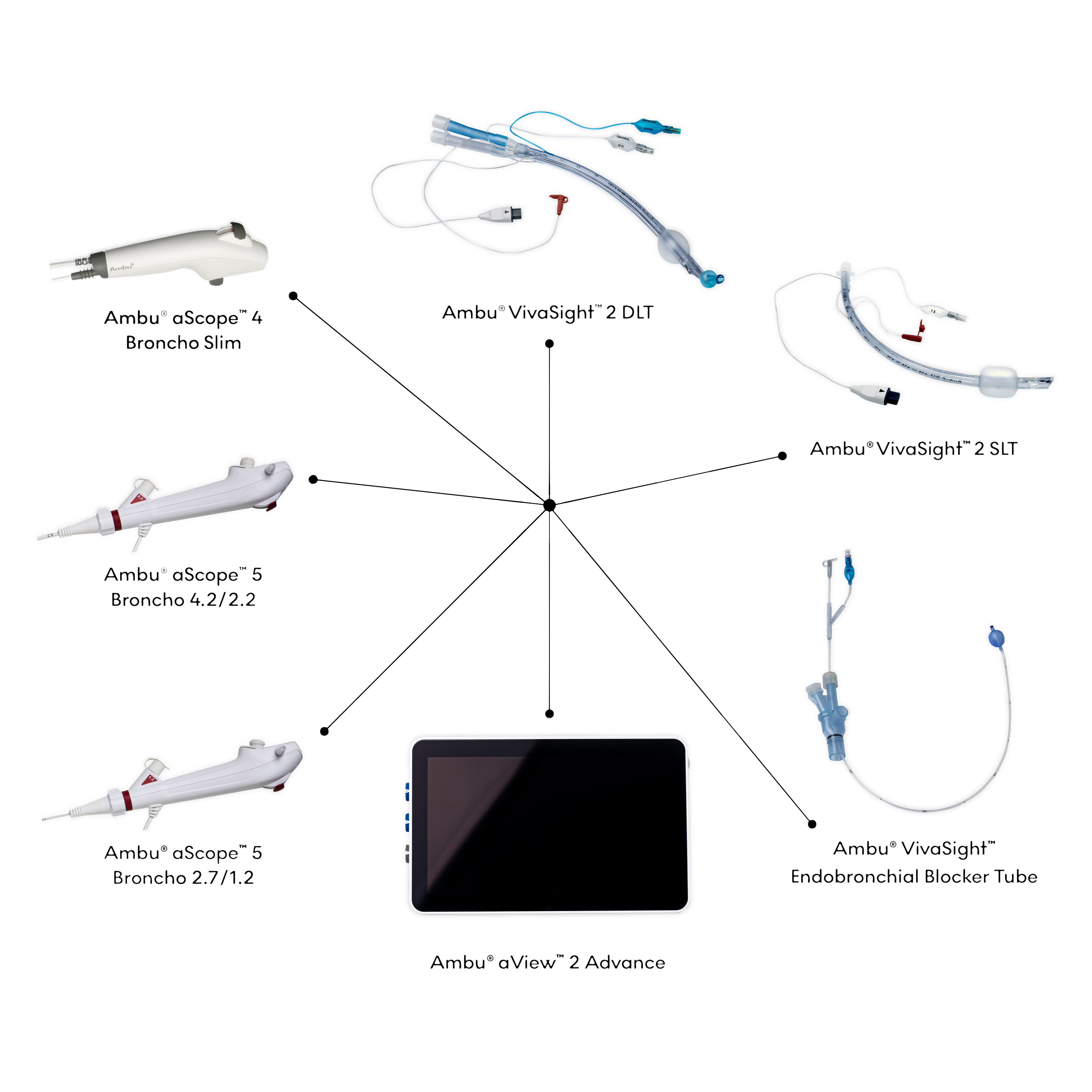
The Ambu OLV solution includes:
Ambu® VivaSight™ 2 DLT
Ambu® VivaSight™ 2 SLT
Ambu® VivaSight™ Endobronchial Blocker tube
Ambu® VivaSight™ Ambu® aScope 5 Broncho 4.2/2.2
Ambu® VivaSight™ Ambu® aScope 5 Broncho 2.7/1.2
Ambu® aScope™ 4 Broncho Slim Bronchoscope
Ambu® aView™ 2 Advance full-HD endoscopy system with Dual View
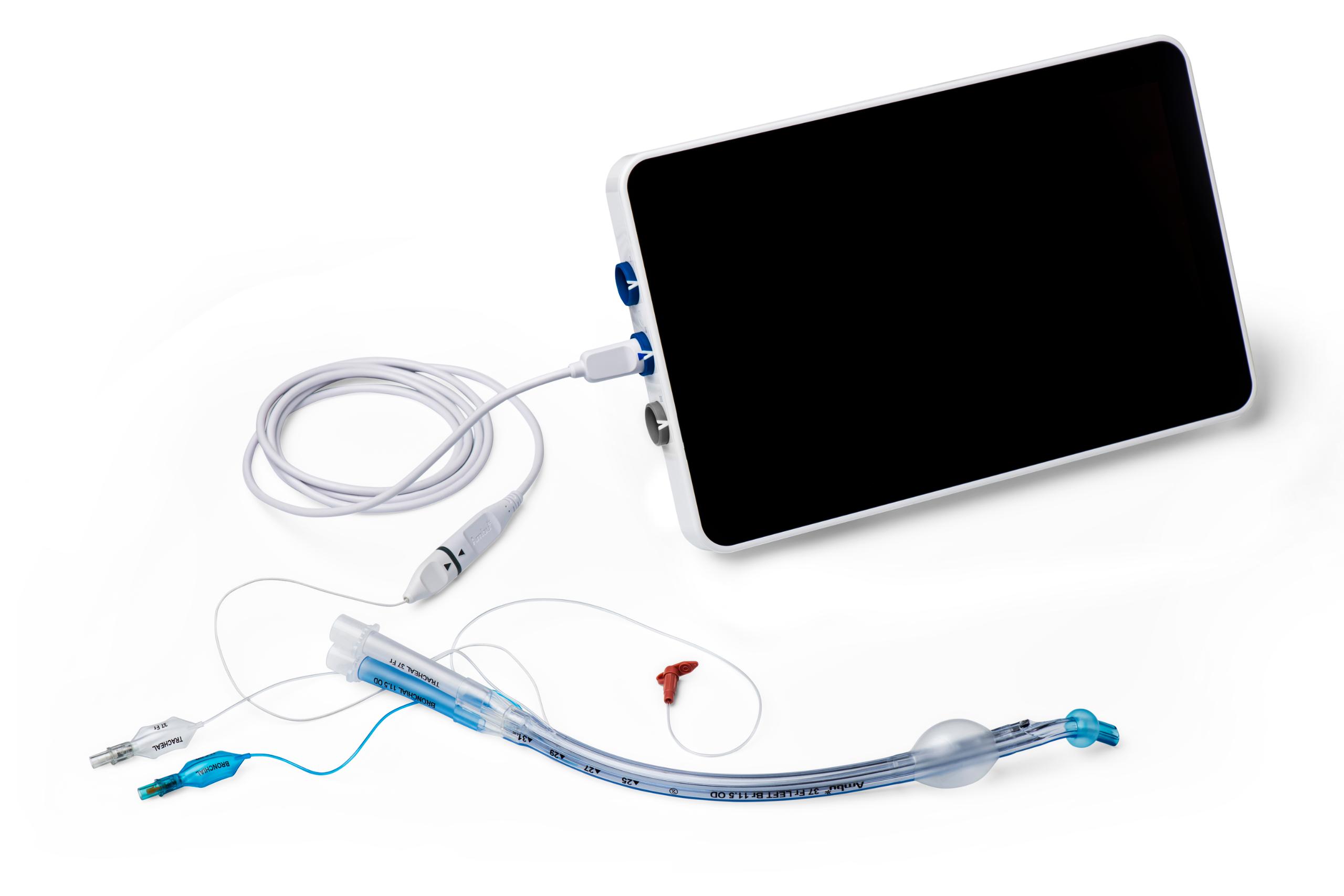
Use VivaSight 2 together with our aView 2 Advance monitor
The combination of the full HD monitor and integrated camera in VivaSight 2 has a number of features, which can help you keep the focus on your patients:
- Integrated Dual View Mode enables you to view real-time video from VivaSight 2 and aScope 4 Broncho Slim simultaneously
- Full HD resolution and advanced image processing ensure high image quality
- Easy recording and storage of images and videos during procedure for documentation and training
Technical specifications
Sizes:
VivaSight 2 DLT: 35 Fr, 37 Fr, 39 Fr & 41 FrFor dimensions, please refer to datasheet
Image sensor:
CMOSLight sources:
2 white LEDsSpare parts
There are no spare parts or accessories for this product.Downloads
Brochures
(5 MB - pdf)
Datasheets
(300.86 KB - pdf)
Instructions for use
(18.98 MB - pdf)
(19.1 MB - pdf)
Supplementary Information
(261.89 KB - pdf)
(6.97 MB - pdf)
August 2021
Note: US: Rx only